The hydrogen value chain
Due to the fact that atomic hydrogen is composed of a proton and an electron, it is not only the first, lightest, and most abundant chemical element in the universe, but above all, it is the most suitable energy vector to support the growing electrification. As a chemical element, it can act as long-term energy storage (contrarily to electricity, and it is not subject to self-discharge like batteries). Furthermore, having only 1 proton and 1 electron can easily produce electricity by combining with oxygen and generating water or by generating oxygen from water via electricity. In fact, atomic hydrogen on earth is mainly present in a combined form with oxygen in the water molecule (H2O) and with carbon in organic compounds (COxHy).
At standard conditions, i.e. at atmospheric pressure and ambient temperature, it exists as the diatomic gas, generally called hydrogen, having the formula H2. It is colourless, odourless, tasteless and flammable gas with the highest energy density by weight (1 kg of H2 contains 120 MJ = 33 KWh = 2.1 kg of natural gas or 2.8 kg of gasoline) but the lowest energy density by volume (gaseous and with a density of 11.123 litres per kg). This means that large volumes and high-pressure storage must be used to meet the same energy demand and storage quantity as gasoline and natural gas.
The drawback of existing in nature only combined with other elements and being the lightest are easily offset by its versatility in terms of production (from water through electricity or from hydrocarbons and organic compounds without electricity), and use (energy storage, heat, electricity-hydrogen-electricity with high efficiency and low emissions through fuel cells with 2-3 times higher efficiencies than engines and turbines). Therefore, it is the best energy vector for electrical storage and decarbonization of hard-to-abate sectors such as transport and heavy industries (petrochemical, steel and iron, etc.).
At standard conditions, i.e. at atmospheric pressure and ambient temperature, it exists as the diatomic gas, generally called hydrogen, having the formula H2. It is colourless, odourless, tasteless and flammable gas with the highest energy density by weight (1 kg of H2 contains 120 MJ = 33 KWh = 2.1 kg of natural gas or 2.8 kg of gasoline) but the lowest energy density by volume (gaseous and with a density of 11.123 litres per kg). This means that large volumes and high-pressure storage must be used to meet the same energy demand and storage quantity as gasoline and natural gas.
The drawback of existing in nature only combined with other elements and being the lightest are easily offset by its versatility in terms of production (from water through electricity or from hydrocarbons and organic compounds without electricity), and use (energy storage, heat, electricity-hydrogen-electricity with high efficiency and low emissions through fuel cells with 2-3 times higher efficiencies than engines and turbines). Therefore, it is the best energy vector for electrical storage and decarbonization of hard-to-abate sectors such as transport and heavy industries (petrochemical, steel and iron, etc.).
Since hydrogen must be produced artificially, although it is colourless, it is classified with over 50 different colours depending on the CO2 emissions of several production methods. The main colours in descending order of emissions are: "brown/black/grey", "purple/pink/red", "blue", "turquoise", "green" if it is obtained, respectively, from lignite/coal/natural gas, from nuclear, from fossils with CO2 capture systems, with the production of solid carbon, and from renewables.
L’idrogeno verde è prodotto tramite un processo elettrochimico chiamato elettrolisi che utilizzando l’elettricità da fonte rinnovabile, rompe la molecola d’acqua separando l’idrogeno dall’ossigeno. La reazione avviene grazie a due elettrodi tra i quali è interposto un elettrolita che può essere solido, elettrolizzatori ad ossido solido (SOEC), o una soluzione in fase liquida, come nel caso delle celle alcaline, o polimerico (PEM). Quando sono sottoposti ad un campo elettrico, le cariche positive (cationi) e negative (anioni) dell’acqua si muovono ordinatamente verso l’elettrodo di carica opposta. Giunti sulla superficie degli elettrodi, i cationi si riducono, acquistando elettroni, mentre gli anioni si ossidano, cedendo elettroni. Avvengono quindi due semireazioni, che nel complesso costituiscono una reazione di ossido riduzione (redox) con la quale si ottiene la decomposizione dell’acqua in idrogeno e ossigeno.
Green hydrogen is produced through an electrochemical process called electrolysis that splits water into hydrogen and oxygen via electricity from renewable sources. This reaction occurs thanks to two electrodes separated by an electrolyte that can be solid, solid oxide electrolyzers (SOEC), or a liquid phase solution, as in the case of alkaline cells, or polymeric (PEM). When subjected to an electric field, the positive (cations) and negative (anion) charges of the water move towards the oppositely charged electrode. Once on the surface of the electrodes, the cations are reduced, acquiring electrons, while the anions oxidize, giving up electrons. Two half-reactions occur as part of a single oxidation-reduction (redox) reaction, decomposing water into hydrogen and oxygen.
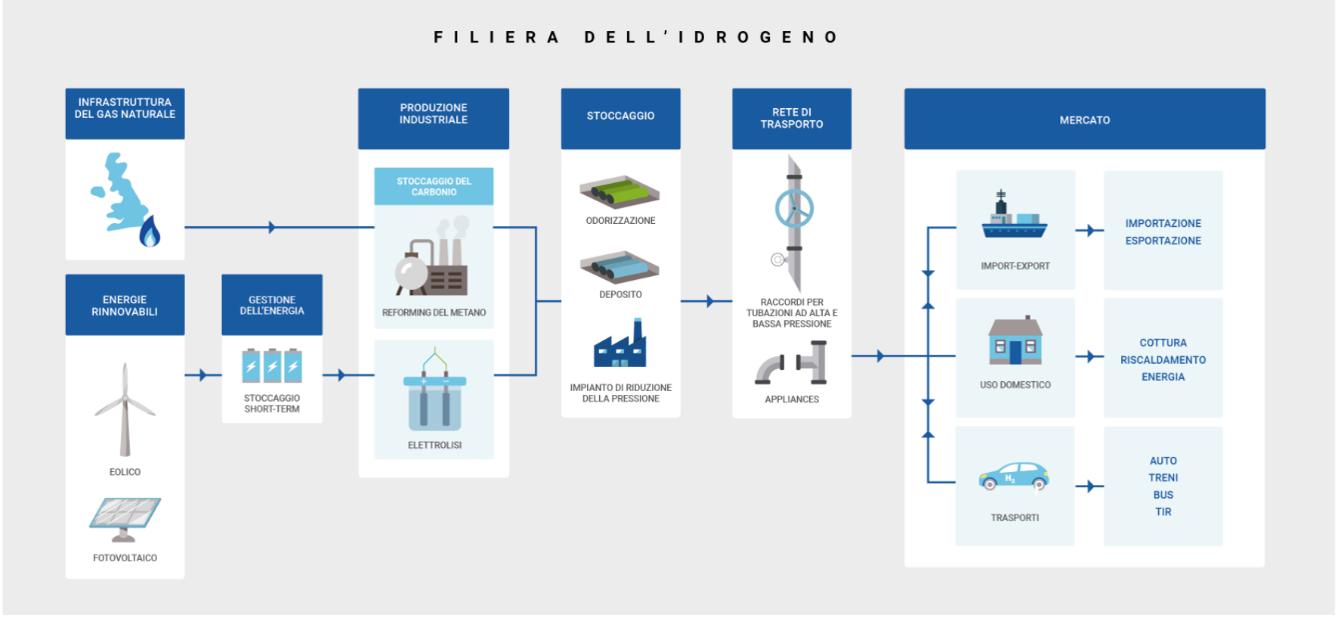
There are also many hydrogen storage, transmission and distribution systems depending on the specific production systems, volumes, flows, distances and uses. In fact, although these systems generally are similar to the one of traditional gas (e.g., compressed gas in small or large cylinders, pipelines carrying pure or mixed gas, such as hydrogen in the natural gas network, liquefied gas), in the case of hydrogen, they are complicated by the low density and by the melting point of -259°C from one side. On the other, they are facilitated by the versatility in production and use that allows hydrogen to be generated and extracted from multiple energy carriers such as methanol, ammonia, etc.
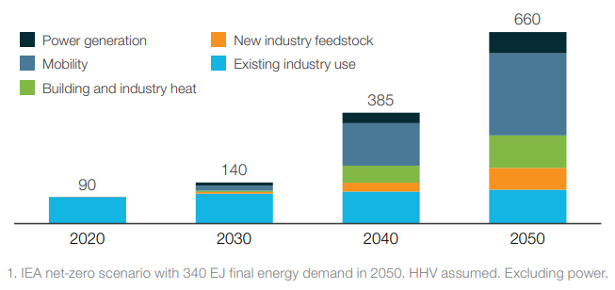
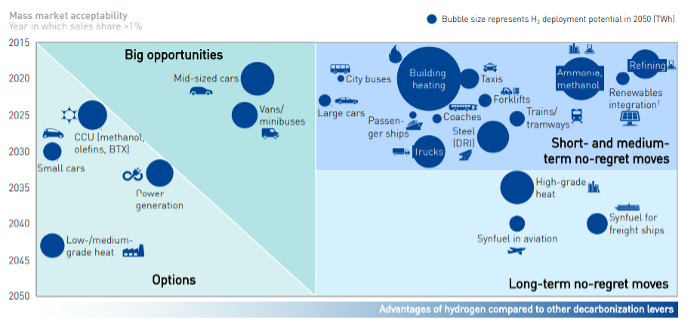
As highlighted in the figures, most of the current demand for hydrogen is dominated by industrial applications. In particular, the four largest consumers are: oil refining (33%), ammonia production (27%), methanol production (11%) and steel production via the direct reduction of iron (3%). However, it is in the transport sector where the most significant increase in demand for hydrogen is expected in the upcoming decades, the value of which could reach 285 million tons in 2050. It is not only road transport that drives demand, hydrogen will play a fundamental role also in the decarbonization of rail transport, especially for the lines not yet electrified and for new lines since further electrification of rail networks is likely to occur. Finally, applications to the maritime and aviation sectors are also being studied. In fact, because of the higher energy content, the zero-emissions (when obtained from renewable energy), and the high conversion efficiencies in the cells compared to the engines make it convenient to use hydrogen in transport even at 2-3 times greater cost than the current fuels.